
Cylinder Inspection Training: Why a Maintained Cylinder Is a Happy Cylinder
Uncover the importance of gas cylinder inspections and training. Dive deep into ensuring safety, boosting efficiency, and prolonging cylinder life.
Have you ever seen a fire at a location that uses or stores pressurized cylinders? Sometimes there is graphic video of a sudden explosion within the fire, throwing shrapnel, knocking people over and creating huge fire balls. This dramatic effect was likely created from a ruptured cylinder within the fire. However, you might see a similar fire, also involving a facility with pressurized cylinders, but only witness smoke and flames, with no fiery explosion. Why was there an exploding cylinder at one fire location and not at the second?
Cylinders are designed to handle pressurized gases. The amount of pressure is based on the material and design thickness of the cylinder. If the cylinder remains within its design specifications it can continue in service for long periods of time. But what if the environment changes? What happens if the cylinder is exposed to extreme environmental conditions, such as an unexpected fire? Will the cylinder rupture due to increased pressure, melt or simply vent the gas? All of this is dependent upon the cylinder material, its condition before the extreme environmental change, the amount of pressure currently in the cylinder and if the safety devices attached to the valve are in proper working order. Even with safety protocols in place, one or two minor issues could become a catastrophic failure.
The three primary materials used to design pressurized cylinders are steel, aluminum and composite; which is primarily composed of carbon fiber. Each of these types of cylinders can be designed to hold large volumes and/or high pressure of gas. Each of them can be susceptible to cuts, corrosion, gouging, dents and dings. These susceptibilities can weaken a cylinder so that it does
not perform or function as designed. The listed concerns thin or weaken the walls in certain areas, making the cylinder weaker and prone to a rupture.
These issues can and should be located during a proper visual inspection. If concerns are located and exceed the allowable limits, the cylinder can be removed from service before a catastrophic event.
But what happens to a cylinder when it is exposed to an environment that is not common, has not been planned, and nobody is in the facility to remove the cylinder from the hazard? This would be the case
involving a fire at a facility or near cylinder storage areas. A fire happens unexpectedly, generally with nobody or a limited number of persons on scene and
can reach temperatures between 1000℉– 2000℉ / 538℃ -1094℃. [1]
The level of temperature has a lasting impact on the
structural integrity of a cylinder. When a steel cylinder reaches temperatures of only 1000℉ / 538℃ the integrity of the metal can be reduced by 50% as shown in the chart.
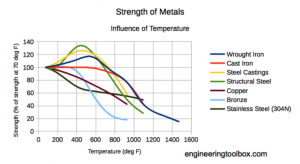
In as little as 800℉ / 427℃ carbon steel can lose 40% of its strength and at 400 degrees the strength can be reduced by 10%[1]

Aluminum is more susceptible to heat exposure. Using a 6061 aluminum alloy as a guide, at 600℉ / 316℃ the cylinder is useless and has little or no structural integrity. At 350℉ / 177℃ the cylinder only has half of its designed pressure integrity. Even at lower temperatures, the cylinder is still susceptible to weakness with temperatures relative to structural integrity. [1] Once the cylinder has been exposed to heat, the structure is permanently weakened and susceptible to further damage.[2]
[1] Thefabricator.com
An empty cylinder won’t rupture in a fire. The cylinder material will become weak when exposed to the extreme temperatures of a fire, but will simply get scorched, or in the case of aluminum, melt. The explosion is caused by the expanding gases within the cylinder. The pressurized gas can normally be contained in an undamaged, non-exposed cylinder. However, when you add heat to a gas it expands. Heat causes gas molecules to move faster, which means the volume of the gas increases. Gases in a fixed volume cannot expand, so increases in surrounding temperature increases the pressure within the cylinder. With this increase of pressure, in a cylinder structure weakened by the heat of a fire, a rupture is likely. A simple explanation of gas expansion can be found here: Pressure and temperature relationship of a gas – The Pressure Law – Pass My Exams: Easy exam revision notes for GSCE Physics
So, if heat weakens the cylinder and expanding gas molecules increase pressure within a weakened cylinder, why don’t all cylinder’s rupture in a fire? That is prevented by the burst disk or over pressure release installed in the cylinder valve. As the gas pressure increases, the burst disc or over pressure release is normally set to activate before a cylinder ruptures. The disc or pressure relief will vent the expanding gases so that the gas does not stress the cylinder itself. The gas vents, preventing the rupture of the cylinder.
So, if the burst disc or the over pressure release vents the gas pressure on a cylinder weakened by heat, why do cylinders rupture during a fire? There could be several reasons, both preventable and non-preventable.
Any time a cylinder is exposed to a fire, it creates a risk of rupture. The desire is that the release valve works, allowing the gas to escape. However, how does a person working around the cylinder, after exposure to a fire, know if the release device activated? There is generally not an indication on the release device showing if it worked. In the case of a burst disc, the copper disc inside the bolt, cannot be seen from the outside.
This author received a call from a fire department a few years back, where two composite cylinders were involved in a fire. Fire personnel asked how they could determine if the burst disc activated, rendering the cylinders safe to handle. They knew the cylinders were pressurized prior to being involved in the fire but had cautions about approaching the cylinders after the fire. It was determined that there was no way to verify the cylinders were pressurized or not. They had law Enforcement come in and shoot the composite cylinders. One cylinder did nothing when shot, meaning the release device activated correctly and there was no pressure in the cylinder. The second cylinder ruptured when impacted by the bullet. Which showed that the release device did not work as expected during the fire.
Any cylinder involved in a fire should be approached with caution. Being involved in a fire will likely weaken the cylinder’s structural integrity. The expanding gas will place even more pressure on the cylinder structure. And it is unknown if the pressure release device worked as designed. This weakened cylinder containing a pressurized gas has a high likelihood of rupturing, even after the fire has been extinguished. Improper handling can cause a catastrophic event.
When dealing with cylinders and fires you need to be cautious during and after a fire. If you know a cylinder has been involved in a fire, but are unsure of its integrity, at a minimum the cylinder should be requalified by a properly licensed requalifier. If it is obvious that the cylinder came in direct contact with a flame, or excessive heat, render it unsafe and dispose it. If the valve is operational, attempt to open and release the gas before moving the cylinder. If the valve is not operational it might be necessary to find unconventional means of reducing the gas pressure within the cylinder. If you are walking around cylinders, after a fire, do not take it for granted that they are harmless. They very well might be looking for a reason to rupture, even after the fire has been extinguished.

I enjoy continuing to build the business based on safety since 1999. CTS focuses on the inspection of high pressure cylinders, the maintaining of the valves and basic maintenance of high pressure compressor systems. CTS stays current in techniques and tools to train both the new and novice employee. We publish articles, update training tools and have created an APP to assist during the inspection process.
#cylinder #safety #hazmat #training #cylinderinspectiontraining #cylindex

Uncover the importance of gas cylinder inspections and training. Dive deep into ensuring safety, boosting efficiency, and prolonging cylinder life.

Is your composite cylinder showing signs of wear? Discover when to seek a professional repair service in our comprehensive guide.

29 CFR 1910.101 intro Handling cryogenic cylinders involves working with extremely low-temperature gases that pose unique safety risks. To ensure the safe handling, storage, and transportation of these hazardous materials,

Introduction Firefighters encounter many risks while on duty, including hazardous materials and high pressure bottles. High pressure bottles are used for a variety of purposes in firefighting, including powering hydraulic